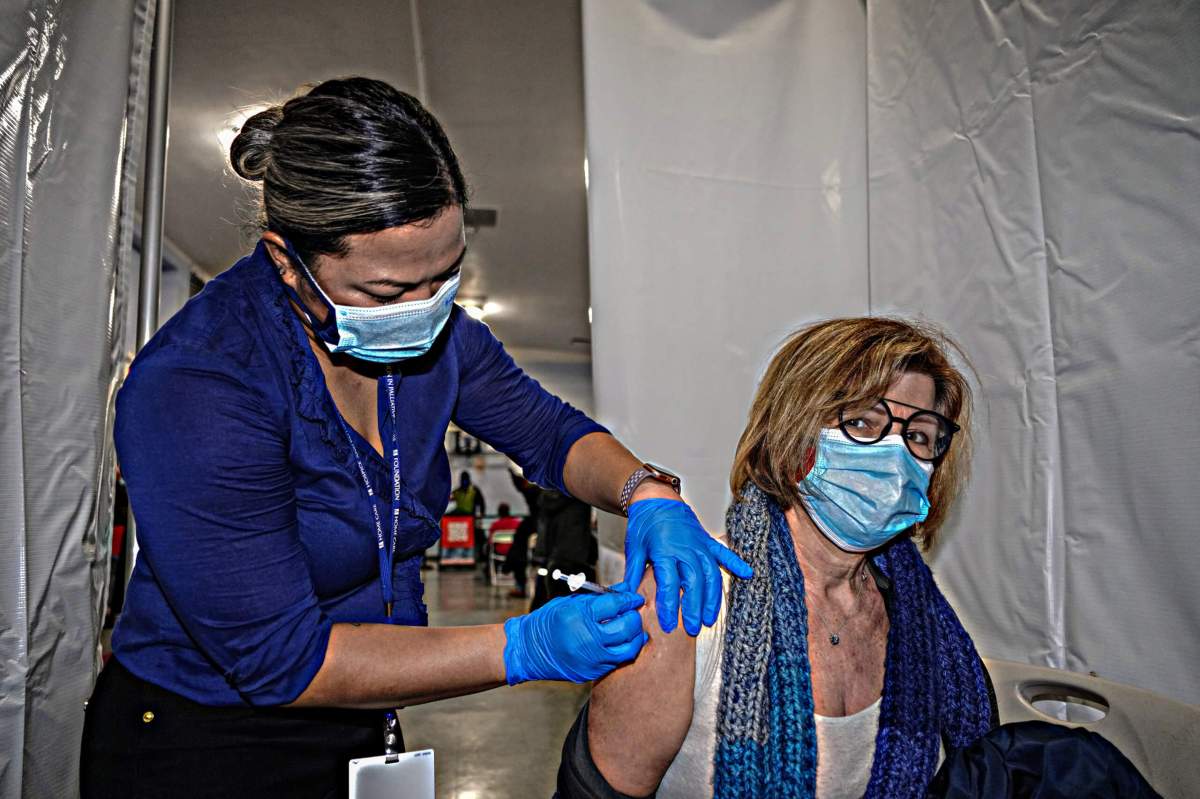
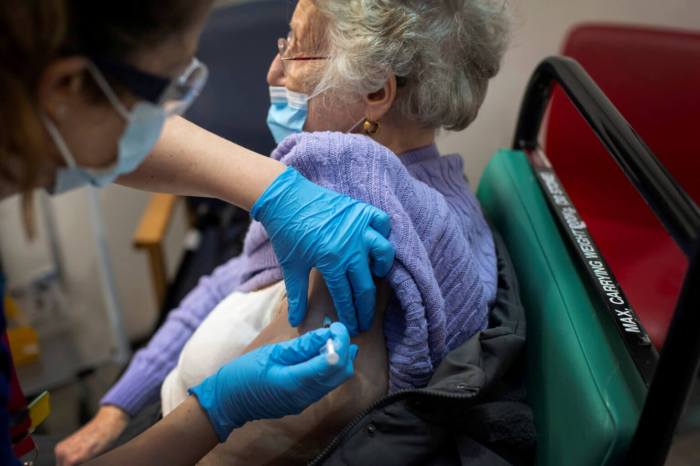
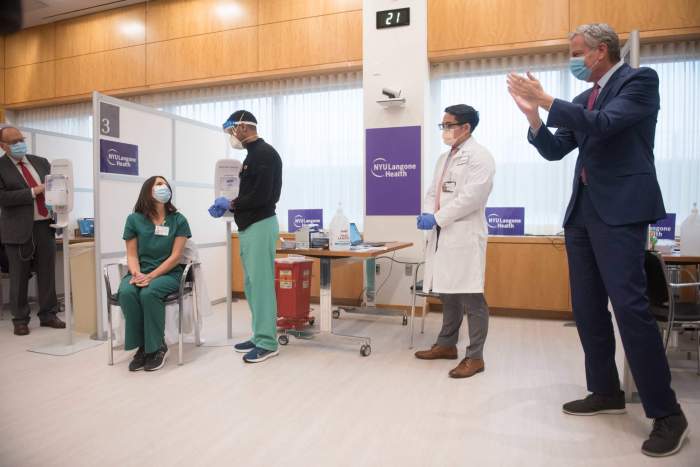
New Yorkers who originally were set to receive the now-paused Johnson & Johnson COVID-19 vaccine will instead roll up their sleeves for the Pfizer vaccine, state Health Commissioner Howard Zucker announced Tuesday.
The announcement comes after the Centers for Disease Control and Prevention and the Food and Drug Administration recommended pausing the use of the one-dose Johnson & Johnson shot to further examine possible side effects, specifically blood clots, that developed in a handful of recipients.
“New York State will follow the CDC and FDA recommendation and pause the use of the Johnson & Johnson vaccine statewide immediately today while these health and safety agencies evaluate next steps,” Zucker said in a statement.
Beginning Tuesday, anyone with an appointment to receive the Johnson & Johnson vaccine will instead get the two-dose Pfizer vaccine, which will remain in use along with the two-dose Moderna COVID-19 vaccine.
The side effects from the Johnson & Johnson vaccine are rare, but are severe. Out of seven million Americans who received the shot, six of them — all women, between ages 18 and 48 — came down with a rare medical condition that induced blood clotting. One of the women died, and another was hospitalized in Nebraska in critical condition, according to The New York Times.
The pause in the use of the Johnson & Johnson COVID-19 vaccine is “out of an abundance of caution,” Zucker noted.
“As the CDC and FDA have said, any adverse events related to the Johnson & Johnson vaccine ‘appear to be extremely rare’ and, ‘People who have received the J&J vaccine who develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider,” Zucker added. “I am in constant contact with the federal government and we will update New Yorkers as more information becomes available.”
A number of prominent New Yorkers have already received the Johnson & Johnson vaccine without major side effects — including Governor Andrew Cuomo, Mayor Bill de Blasio and City Health Commissioner Dr. Dave Chokshi.
Even with the Johnson & Johnson vaccine put on pause for the time being, de Blasio expressed confidence Tuesday that the shot is largely safe.
“We obviously had faith in it for our own selves, our own families. And I think that that faith has been proven, warranted to date,” the mayor said. “But again anytime you see something new you have to look into it.”
Chokshi, meanwhile, said the Pfizer and Moderna vaccines would continue to be distributed around the city while the J&J vaccine is put on ice.
“That’ll help us beat back the pandemic as we assess the J&J vaccine. And second, while these events are extremely rare based on what we know now, the federal government has recommended that we take this step,” the commissioner said. “The city is proceeding out of an abundance of caution to pause the administering as we’ve described. This is deliberately being termed as a pause because it’s a chance to step back and give our partners at the CDC a chance to investigate the cases.”
Chokshi said the vaccine system, overall, is “working exactly as it should.”
“And the actions we take locally reflect how seriously we take signals from our warning system,” he added.
Still, Manhattan City Council Member Mark Levine, who chairs the body’s Health Committee, called the pause “a major blow” to the vaccination effort, particularly in the pursuit of vaccine equity — getting the shots to communities that have been traditionally ignored.
“The timing of this disruption is particularly unfortunate, at a moment when vaccination is transitioning from those who have been motivated to find an appointment on the web, to those the City has to work harder to reach,” Levine said in a statement. “We need to redouble our efforts to vaccinate those being left behind in New York City.”
Vaccine differences
Unlike the Johnson & Johnson vaccine — which, like traditional vaccines, introduce a weakened version of a virus into the body to boost an immune response — the Pfizer and Moderna shots are mRNA-based vaccines which involve the use of weakened copies of the COVID-19 protein in order to train the body to fight the virus. These vaccines do not alter the body’s genetics.
According to the CDC, the Pfizer vaccine has been “100% effective” in preventing severe COVID-19 disease. An FDA study found the efficacy rate to be 95.3%. The Moderna vaccine efficacy rate, meanwhile, was 94.3%.
The most common side effects of the Pfizer and Moderna vaccines have been brief periods of fever, fatigue, aches and pain in the injection site, usually within 24 to 48 hours after the dose is administered.
With reporting by Ariama C. Long
This story was updated on April 13 at 1:25 p.m.