By Carl O’Donnell and Michael Erman
U.S. President Donald Trump and the head of the Centers for Disease Control and Prevention (CDC) this week disagreed about when a COVID-19 vaccine would become widely available. Trump has said one could initially be available by the Nov. 3 election, while the CDC director said vaccines were likely to reach the general public around mid-2021, an assessment more in line with most experts.
WHAT DOES IT MEAN FOR A VACCINE TO GENERALLY AVAILABLE?
General availability is when every American who wants the vaccine can get it. There are currently no COVID-19 vaccines approved by U.S. regulators, although a handful are in late-stage trials to prove they are safe and effective.
Experts estimate that at least 70% of roughly 330 million Americans would need to be immune through a vaccine or prior infection to achieve what is known as herd immunity, which occurs when enough people are immune to prevent the spread of the virus to those unable to get a vaccine.
HOW LONG BEFORE VACCINE PRODUCTION IS FULLY RAMPED UP?
Most vaccines in development will require two doses per person.
The CDC anticipates that 35 million to 45 million doses of vaccines from the first two companies to receive authorization will be available in the United States by the end of this year. The current front runners are Pfizer Inc and Moderna Inc.
Drugmakers have been more ambitious with their calculations. AstraZeneca Plc has said it could deliver as many as 300 million doses of its experimental vaccine in the United States by as early as October. Pfizer and German partner BioNTech SE have said they expect to have 100 million doses available worldwide by the end of 2020, but did not specify how much of that was earmarked for the United States. Moderna on Friday said it is on track to make around 20 million doses by the end of the year and between 500 million and 1 billion doses a year beginning in 2021.
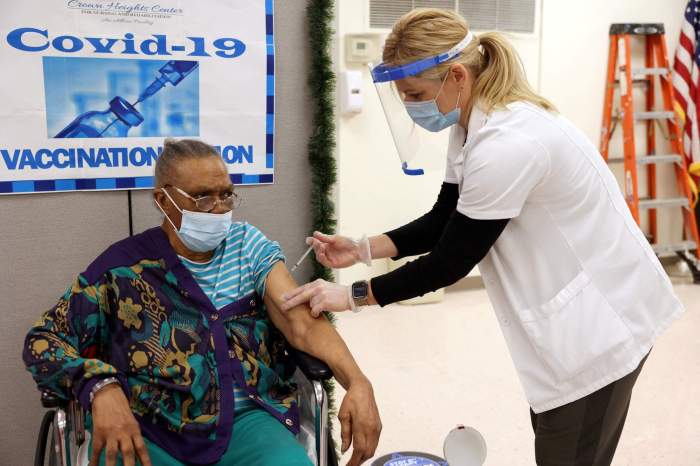
Obtaining enough doses to inoculate everyone in the United States will likely take until later in 2021. CDC Director Robert Redfield told a congressional hearing on Wednesday that vaccines may not be widely available to everyone in the United States until the second or third quarter of next year.
WHO WOULD GET AN APPROVED VACCINE FIRST?
The CDC decision will likely broadly follow recommendations from the National Academies of Sciences, Engineering and Medicine. [L1N2FY1CX] The CDC has said the earliest inoculations may go to healthcare workers, people at increased risk for severe COVID-19, and essential workers.
It is unclear when a vaccine will be available for children as major drugmakers have yet to include them in late-stage trials. Pfizer and BioNTech have filed with regulators seeking to start recruiting volunteers as young as 16 for vaccine studies.
WHICH COMPANIES WILL LIKELY ROLL OUT A VACCINE QUICKLY?
Pfizer has said it could have compelling evidence that its vaccine works by the end of October. Moderna says it could have similar evidence in November. The vaccines would first need to be approved or authorized for emergency use by U.S. regulators.
Drugmakers have already started manufacturing supplies of their vaccine candidates to be ready as soon as they get the go ahead. The U.S. Department of Defense and the CDC plan to start distribution of vaccines within 24 hours of regulatory authorization.
Several drugmakers including Pfizer, AstraZeneca, Johnson & Johnson and Novavax Inc have all said they expect to produce at least 1 billion doses of their vaccines next year if they get regulatory authorization.
Sanofi SA and GlaxoSmithKline Plc are also working on developing a vaccine they say could be authorized next year.