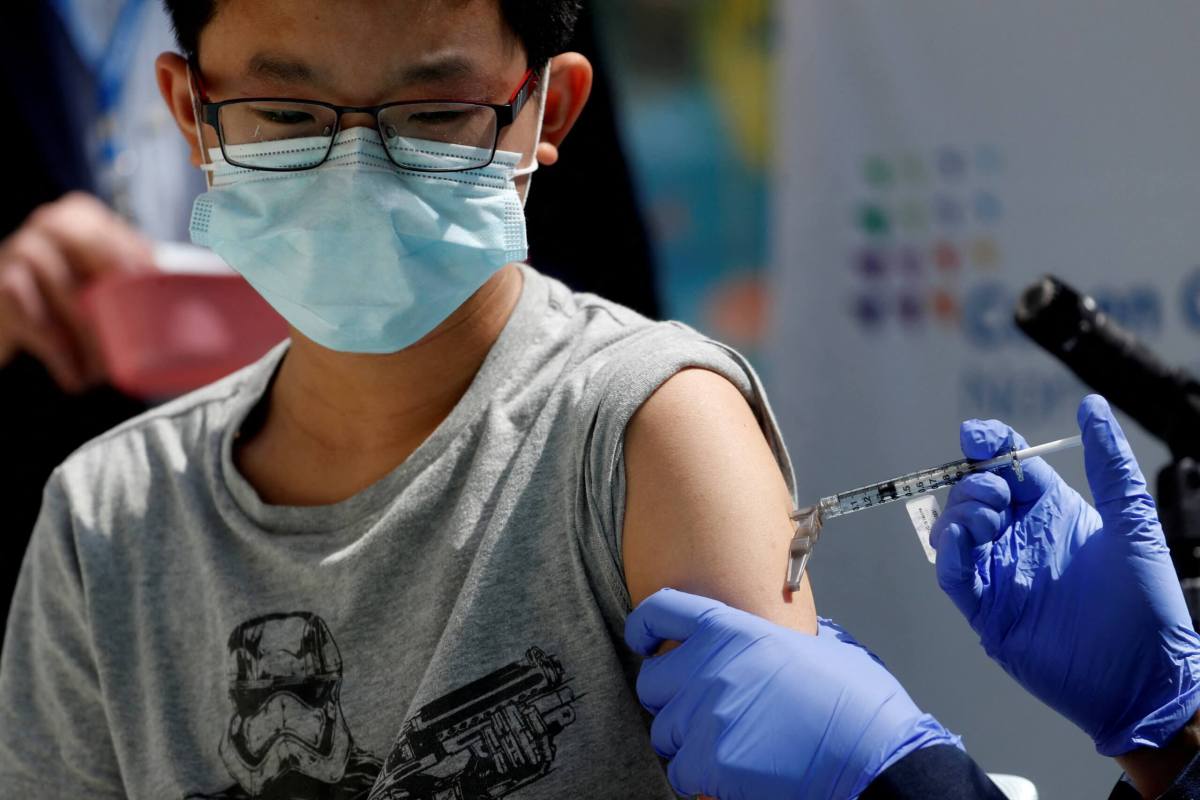
The U.S. Food and Drug Administration on Monday authorized the use of a third dose of the Pfizer and BioNTech COVID-19 vaccine for children aged between 12 and 15 years, and narrowed the time for all booster shots to 5 months from 6 months after primary doses.
The agency also authorized a third shot in children aged 5 through 11 years who are immunocompromised.
The FDA said it reviewed published data and real world evidence on the safety of booster doses provided by the Israeli Ministry of Health including data from over 6,300 individuals 12-to-15 years of age who received a Pfizer shot.
Global COVID-19 cases are surging due to the Omicron variant and health authorities have warned that its extremely high transmissibility could overwhelm many health systems.
Laboratory tests have shown that two doses of the Pfizer-BioNTech and Moderna vaccines generate low immune responses against Omicron, while boosters appear to be protective against the highly-mutated variant.